
Breaking News
 Grand Theft World Podcast 273 | Goys 'R U.S. with Guest Rob Dew
Grand Theft World Podcast 273 | Goys 'R U.S. with Guest Rob Dew
 Anchorage was the Receipt: Europe is Paying the Price… and Knows it.
Anchorage was the Receipt: Europe is Paying the Price… and Knows it.
 The Slow Epstein Earthquake: The Rupture Between the People and the Elites
The Slow Epstein Earthquake: The Rupture Between the People and the Elites
 Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
Top Tech News
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
Chemists have successfully produced fuels using water, CO2 and visible light through artificial phot
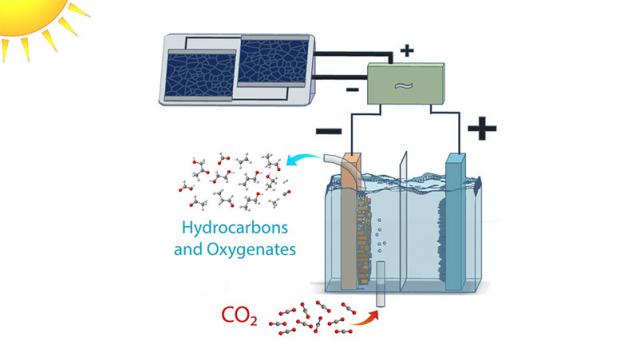
Photochemical conversion of CO2 into fuels has promise as a strategy for storage of intermittentsolar energy in the form of chemical bonds. However, higher-energy-value hydrocarbonsare rarely produced by this strategy, because of kinetic challenges. Here we demonstrate a strategy for green-light-driven synthesis of C1-C3 hydrocarbons from CO2 and H2O. In this approach, plasmonic excitation of Au nanoparticles produces a charge-rich environment at the nanoparticle/solution interface conducive for CO2 activation, while an ionic liquid stabilizes charged intermediates formed at this interface, facilitating multistep reduction and C-C coupling. Methane, ethylene, acetylene, propane, and propene are photosynthesized with a C2+ selectivity of ~50% under the most optimal conditions. Hydrocarbon turnover exhibits a volcano relationship as a function of the ionic liquid concentration, the kinetic analysis of which coupled with density functional theory simulationsprovides mechanis



