
Breaking News
 IT'S OVER: Banks Tap Fed for $17 BILLION as Silver Shorts Implode
IT'S OVER: Banks Tap Fed for $17 BILLION as Silver Shorts Implode
SEMI-NEWS/SEMI-SATIRE: December 28, 2025 Edition
 China Will Close the Semiconductor Gap After EUV Lithography Breakthrough
China Will Close the Semiconductor Gap After EUV Lithography Breakthrough
 The Five Big Lies of Vaccinology
The Five Big Lies of Vaccinology
Top Tech News
 EngineAI T800: Born to Disrupt! #EngineAI #robotics #newtechnology #newproduct
EngineAI T800: Born to Disrupt! #EngineAI #robotics #newtechnology #newproduct
 This Silicon Anode Breakthrough Could Mark A Turning Point For EV Batteries [Update]
This Silicon Anode Breakthrough Could Mark A Turning Point For EV Batteries [Update]
 Travel gadget promises to dry and iron your clothes – totally hands-free
Travel gadget promises to dry and iron your clothes – totally hands-free
 Perfect Aircrete, Kitchen Ingredients.
Perfect Aircrete, Kitchen Ingredients.
 Futuristic pixel-raising display lets you feel what's onscreen
Futuristic pixel-raising display lets you feel what's onscreen
 Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
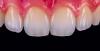 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Process for producing ammonia that generates electricity instead of consuming energy...

Nearly a century ago, German chemist Fritz Haber won the Nobel Prize in Chemistry for a process to generate ammonia from hydrogen and nitrogen gases. The process, still in use today, ushered in a revolution in agriculture, but now consumes around one percent of the world's energy to achieve the high pressures and temperatures that drive the chemical reactions to produce ammonia.
Today, University of Utah chemists publish a different method, using enzymes derived from nature, that generates ammonia at room temperature. As a bonus, the reaction generates a small electrical current.
Although chemistry and materials science and engineering professor Shelley Minteer and postdoctoral scholar Ross Milton have only been able to produce small quantities of ammonia so far, their method could lead to a less energy-intensive source of the ammonia, used worldwide as a vital fertilizer.



