
Breaking News
 Iran Regime Kills Protesters as Unrest and Calls for Regime Change Spread Nationwide
Iran Regime Kills Protesters as Unrest and Calls for Regime Change Spread Nationwide
 Trump, Treason, and the New York Times
Trump, Treason, and the New York Times
 Democrat idiocy at work in San Francisco
Democrat idiocy at work in San Francisco
 BREAKING THROUGH Tesla AI in 2026
BREAKING THROUGH Tesla AI in 2026
Top Tech News
 Laser weapons go mobile on US Army small vehicles
Laser weapons go mobile on US Army small vehicles
 EngineAI T800: Born to Disrupt! #EngineAI #robotics #newtechnology #newproduct
EngineAI T800: Born to Disrupt! #EngineAI #robotics #newtechnology #newproduct
 This Silicon Anode Breakthrough Could Mark A Turning Point For EV Batteries [Update]
This Silicon Anode Breakthrough Could Mark A Turning Point For EV Batteries [Update]
 Travel gadget promises to dry and iron your clothes – totally hands-free
Travel gadget promises to dry and iron your clothes – totally hands-free
 Perfect Aircrete, Kitchen Ingredients.
Perfect Aircrete, Kitchen Ingredients.
 Futuristic pixel-raising display lets you feel what's onscreen
Futuristic pixel-raising display lets you feel what's onscreen
 Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
A Novel Aluminum–Graphite Dual-Ion Battery
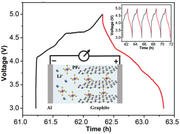
A packaged aluminum–graphite battery is estimated to deliver an energy density of ≈150 Wh kg−1 at a power density of ≈1200 W kg−1, which is ≈50% higher than most commercial lithium ion batteries.
Lithium ion batteries based on cation intercalation have been powering the increasingly mobile society for decades.[1] In a conventional lithium ion battery, the intercalation of lithium ions in both cathode (i.e., LiCoO2, LiFePO4) and anode (i.e., graphite, silicon) materials have been thoroughly studied, while the utilization of the anions in the electrolyte has drawn much less attention.[2] In fact, the phenomenon of anion intercalate into graphite by chemical or electrochemical means was discovered and proposed as a possible positive electrode for batteries by Rüdorff and Hofmann in 1938.[3] However, the anion intercalation was achieved by using high concentration acid solution as electrolyte, this brought serious safety issue that hindered its application.[4] In the 1990s, soon after the commercial application of lithium ion battery, Carlin et al. reported dual graphite intercalating molten electrolyte batteries that realized the application of anion intercalated graphite as positive electrode in batteries by using room temperature ionic liquids as electrolyte.[5] In the following decades, continuous progresses have been made in anion intercalated graphite based dual carbon batteries, such as investigation of anion intercalation in non-aqueous electrolyte, in situ characterization of the staged anion intercalation process, and systematic study of the intercalation of different anions into graphite.[6]



