
Breaking News
 James O'Keefe: My entire speech at AmericaFest 2025. We're not stopping. Join us to expose..
James O'Keefe: My entire speech at AmericaFest 2025. We're not stopping. Join us to expose..
 U.S. vs. Chinese Military Comparison – Focus on Asia-Taiwan Scenario
U.S. vs. Chinese Military Comparison – Focus on Asia-Taiwan Scenario
 DoJ Sues Four More States for Failing To Produce Voter-roll Data
DoJ Sues Four More States for Failing To Produce Voter-roll Data
 World's Largest Aviation Giant Abandons Google Over Security Concerns
World's Largest Aviation Giant Abandons Google Over Security Concerns
Top Tech News
 Perfect Aircrete, Kitchen Ingredients.
Perfect Aircrete, Kitchen Ingredients.
 Futuristic pixel-raising display lets you feel what's onscreen
Futuristic pixel-raising display lets you feel what's onscreen
 Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
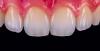 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
 A microbial cleanup for glyphosate just earned a patent. Here's why that matters
A microbial cleanup for glyphosate just earned a patent. Here's why that matters
 Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Can diamonds burn?

Diamonds are forever, or so the slogan goes. But with the proper application of heat and enough oxygen, a diamond can go up in smoke.
Diamonds are carbon, just like coal. It takes a bit more to get them burning and keep them burning than coal, but they will burn, as numerous YouTube demonstrations will attest. The trick is to create the right conditions so that a solid diamond can react with the oxygen required to fuel a fire.
"You have to convert that solid [carbon] into a gas form, so it can react with the air to make a flame," said Rick Sachleben, a retired chemist and member of the American Chemical Society.
The best way to do that? Heat — and lots of it. In room temperature air, diamonds ignite at around 1,652 degrees Fahrenheit (900 degrees Celsius), according to West Texas A&M University physicist Christopher Baird. For comparison, a high-volatile coal (coal containing a relatively high amount of easily released gases) ignites at about 1,233 F (667 C), whereas wood ignites at 572 F (300 C) or less, depending on the type.
When first heated, a diamond will glow red, then white. The heat enables a reaction between the surface of the diamond and the air, converting the carbon to the colorless and odorless gas carbon monoxide (a carbon atom plus an oxygen atom).
"The carbon plus the oxygen to make carbon monoxide generates heat; the carbon monoxide reacting with the oxygen generates more heat; the rising heat causes the carbon monoxide to move away, so more oxygen is brought in," he told Live Science.

 Advanced Propulsion Resources Part 1 of 2
Advanced Propulsion Resources Part 1 of 2

