
Breaking News
 Jimmy Dore Compares Trump's Endorsement of Overthrowing Libya to His Actions in Venezuela
Jimmy Dore Compares Trump's Endorsement of Overthrowing Libya to His Actions in Venezuela
 Pfizer mRNA Found in Over 88% of Human Placentas, Sperm, and Blood -- and in 50% of Unvaccinated...
Pfizer mRNA Found in Over 88% of Human Placentas, Sperm, and Blood -- and in 50% of Unvaccinated...
 "All real footage, no CGI, no AI, no video speed-up." Looks fake to me – their robot…
"All real footage, no CGI, no AI, no video speed-up." Looks fake to me – their robot…
 This Immigrant Admitted Their Plan for White PeopIe in America And It's Far Worse Than Most Thou
This Immigrant Admitted Their Plan for White PeopIe in America And It's Far Worse Than Most Thou
Top Tech News
 Build a Greenhouse HEATER that Lasts 10-15 DAYS!
Build a Greenhouse HEATER that Lasts 10-15 DAYS!
 Look at the genius idea he came up with using this tank that nobody wanted
Look at the genius idea he came up with using this tank that nobody wanted
 Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
 Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
 Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
 We've wiretapped the gut-brain hotline to decode signals driving disease
We've wiretapped the gut-brain hotline to decode signals driving disease
 3D-printable concrete alternative hardens in three days, not four weeks
3D-printable concrete alternative hardens in three days, not four weeks
 Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
Immortal Monkeys? Not Quite, But Scientists Just Reversed Aging With 'Super' Stem Cells
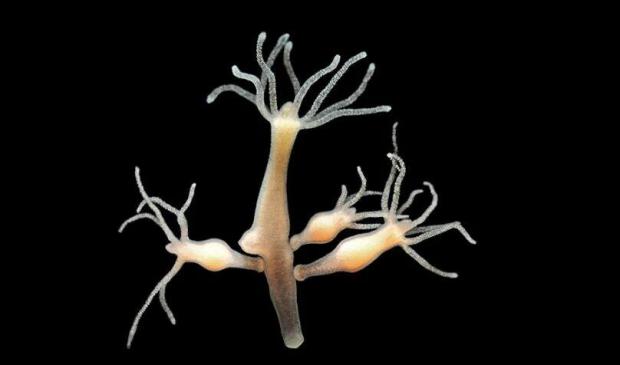
By fortifying human stem cells with a gene long linked to longevity, they rejuvenated aged monkeys - improving memory, protecting bones, calming inflammation, and restoring youthful activity across dozens of organs.
The work, while still in animals, is among the most compelling demonstrations yet that aging in primates might be reversible.
The Science Behind the Breakthrough
At the heart of the study are mesenchymal progenitor cells (MPCs) - a type of stem-like cell found in bone marrow and connective tissues. These cells act as the body's maintenance crew, capable of turning into bone, cartilage, fat, and muscle cells, while also secreting factors that help nearby tissues repair themselves.
But like all cells, MPCs age with us and eventually succumb to senescence a state of permanent retirement. Senescent cells don't divide anymore. Worse, they pump out inflammatory molecules, scar tissue signals, and other "toxic chatter" that accelerate aging in neighboring cells. In effect, senescent cells spread decline.
Upgrading the Repair System with FoxO3
To overcome this exhaustion, researchers turned to FoxO3, a protein known as a longevity gene regulator. In healthy young cells, FoxO3 acts like a switchboard operator, turning on DNA repair pathways, antioxidant defenses, and stress-resistance programs. In older cells, FoxO3 activity wanes - leaving them vulnerable to damage.
Hydra, a freshwater organism capable of regenerating indefinitely, rely heavily on FoxO to keep their stem cells active. Humans share this same protein, and genetic studies link variants of FOXO3 to exceptional longevity in people.
The Chinese Academy of Sciences team genetically engineered MPCs so that FoxO3 would stay permanently active inside the nucleus, constantly flipping on protective genes.
The researchers engineered senescence-resistant cells - "SRCs" - by altering genes that control DNA repair, stress resistance, and mitochondrial function. These fortified cells were then transplanted into elderly macaques whose age roughly corresponds to a human in their 60s or 70s.
They found that SRC treatment mitigated age-related brain shrinkage, and rejuvenated multiple organs and tissues.
Put simply: MPCs provided the hardware - the body's natural repair crew - while FoxO3 was the software upgrade that made them resistant to aging.

 First totally synthetic human brain model has been realized
First totally synthetic human brain model has been realized Mach-23 potato gun to shoot satellites into space
Mach-23 potato gun to shoot satellites into space

