
Breaking News
 The Epstein Files: A Peek Behind the Dark Curtain, Pt. 1
The Epstein Files: A Peek Behind the Dark Curtain, Pt. 1
 Make Your Own Herbal Tea Blends and Save $$$
Make Your Own Herbal Tea Blends and Save $$$
 Prince Andrews Arrested, Trump Releasing Alien Files, Supreme Court Tariff Decision...
Prince Andrews Arrested, Trump Releasing Alien Files, Supreme Court Tariff Decision...
Top Tech News
 New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
 AI-enhanced stethoscope excels at listening to our hearts
AI-enhanced stethoscope excels at listening to our hearts
 Flame-treated sunscreen keeps the zinc but cuts the smeary white look
Flame-treated sunscreen keeps the zinc but cuts the smeary white look
 Display hub adds three more screens powered through single USB port
Display hub adds three more screens powered through single USB port
 We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
39-Year-Old Becomes First US Patient to Receive Innovative Artificial Heart Prosthetic
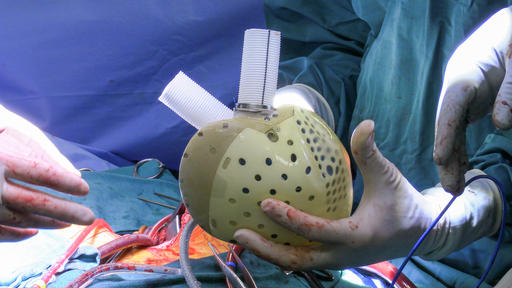
The artificial heart was developed by a French company, CARMAT and has been approved for use and sale in Europe.
Last year, the company received U.S. FDA approval to begin studies and enroll 10 patients with end-stage biventricular heart failure—people who are suffering on the waiting list for a heart donor—and offer a life-saving bridge before transplant.
"We are encouraged that our patient is doing so well after the procedure Monday," said Dr. Carmelo Milano, a transplant surgeon and the principal investigator of the device study at Duke. "As we evaluate this device, we are both excited and hopeful that patients who otherwise have few to no options could have a lifeline."
The North Carolina patient, Matthew Moore, is just 39-years-old and was referred to Duke in June after a sudden, unexpected diagnosis of heart failure. Moore and his wife, Rachel, recently adopted their two-year-old foster son, Marshall, and arrived at Duke expecting only to undergo heart bypass surgery.

 Free Grocery Stores in NYC?
Free Grocery Stores in NYC? 

