
Breaking News
 U.S. evacuates 20,000 citizens as Middle East war intensifies
U.S. evacuates 20,000 citizens as Middle East war intensifies
 MAJOR GLOBAL ECONOMIC CRISIS UPDATE: The Closure Of The Strait of Hormuz For Just 4 Days...
MAJOR GLOBAL ECONOMIC CRISIS UPDATE: The Closure Of The Strait of Hormuz For Just 4 Days...
 Counting The Costs: Another War Is Not What America Needed
Counting The Costs: Another War Is Not What America Needed
 Researchers Train Bacteria to Consume Tumors from the Inside Out
Researchers Train Bacteria to Consume Tumors from the Inside Out
Top Tech News
 The Pentagon is looking for the SpaceX of the ocean.
The Pentagon is looking for the SpaceX of the ocean.
 Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
 Scientists at Rice University have developed an exciting new two-dimensional carbon material...
Scientists at Rice University have developed an exciting new two-dimensional carbon material...
 Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
 ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
 DARPA Launches New Program Generative Optogenetics, GO,...
DARPA Launches New Program Generative Optogenetics, GO,...
 Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
 Ordering a Tiny House from China, what's the real COST?
Ordering a Tiny House from China, what's the real COST?
 New video may offer glimpse of secret F-47 fighter
New video may offer glimpse of secret F-47 fighter
 Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
AstraZeneca Coronavirus Vaccine to Be Pulled Worldwide for 'Commercial' Reasons
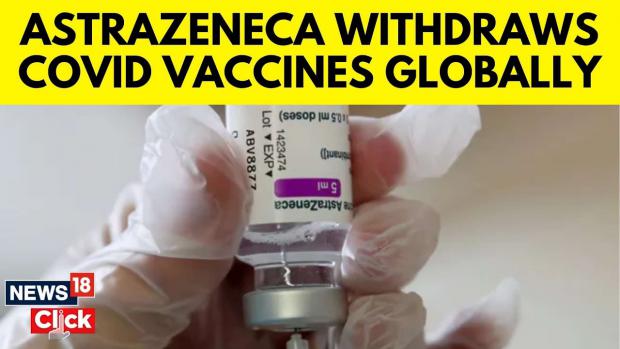
The Oxford-AstraZeneca coronavirus vaccine will be withdrawn worldwide just months after the firm admitted that the jab causes a rare but dangerous side effect, however, AstraZeneca insists that the decision to pull the vaccine was purely made for commercial reasons.
AstraZeneca pulled its "marketing authorisation" for its COVID-19 vaccine, known commercially as Vaxzevria, in the European Union on Tuesday and plans to do so in the UK and other countries where it was deployed in the coming months. The decision will not impact the United States, where the jab never received approval to go to market.
The pharmaceutical giant insisted that the decision was made for commercial considerations, saying that the vaccine is no longer being manufactured and that more recent vaccines have been developed to confront novel variants of the Chinese virus.



