
Breaking News
 Audio + English transcript from the closed-door July 9, 2025 court hearing in the case against...
Audio + English transcript from the closed-door July 9, 2025 court hearing in the case against...
 Trump: Obama started this WHOLE thing! (6 mins on it from the Maria B interview)
Trump: Obama started this WHOLE thing! (6 mins on it from the Maria B interview)
 Provoked: How Washington Started the New Cold War with Russia and the Catastrophe in Ukraine
Provoked: How Washington Started the New Cold War with Russia and the Catastrophe in Ukraine
 US Politics Is Just Nonstop Fake Revolutions Now
US Politics Is Just Nonstop Fake Revolutions Now
Top Tech News
 3D Printed Aluminum Alloy Sets Strength Record on Path to Lighter Aircraft Systems
3D Printed Aluminum Alloy Sets Strength Record on Path to Lighter Aircraft Systems
 Big Brother just got an upgrade.
Big Brother just got an upgrade.
SEMI-NEWS/SEMI-SATIRE: October 12, 2025 Edition
 Stem Cell Breakthrough for People with Parkinson's
Stem Cell Breakthrough for People with Parkinson's
 Linux Will Work For You. Time to Dump Windows 10. And Don't Bother with Windows 11
Linux Will Work For You. Time to Dump Windows 10. And Don't Bother with Windows 11
 XAI Using $18 Billion to Get 300,000 More Nvidia B200 Chips
XAI Using $18 Billion to Get 300,000 More Nvidia B200 Chips
 Immortal Monkeys? Not Quite, But Scientists Just Reversed Aging With 'Super' Stem Cells
Immortal Monkeys? Not Quite, But Scientists Just Reversed Aging With 'Super' Stem Cells
 ICE To Buy Tool That Tracks Locations Of Hundreds Of Millions Of Phones Every Day
ICE To Buy Tool That Tracks Locations Of Hundreds Of Millions Of Phones Every Day
 Yixiang 16kWh Battery For $1,920!? New Design!
Yixiang 16kWh Battery For $1,920!? New Design!
 Find a COMPATIBLE Linux Computer for $200+: Roadmap to Linux. Part 1
Find a COMPATIBLE Linux Computer for $200+: Roadmap to Linux. Part 1
Transhumanist Scientists Create Embryos From Skin Cells And Sperm
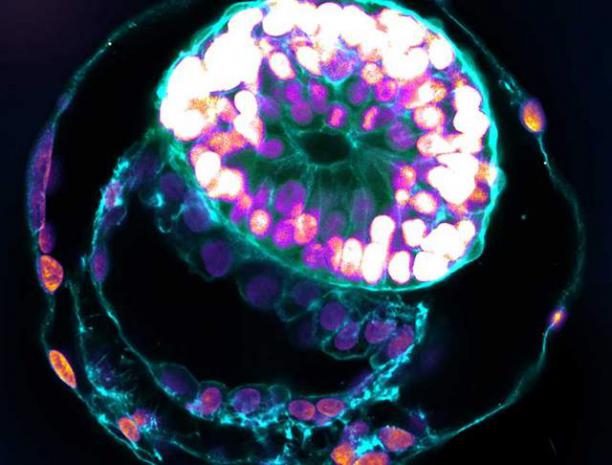
The work is a laboratory demonstration that shows what might eventually be possible for people who cannot produce viable eggs, though substantial scientific hurdles remain.
The team at Oregon Health & Science University used a technique called somatic cell nuclear transfer, where a skin cell's nucleus is placed into a donated egg that has had its own genetic material removed. After fertilization with sperm, these reconstructed cells produced embryos that developed for several days. While purely experimental and years from clinical use, the research published in Nature Communications shows an early demonstration of mitomeiosis, the team's term for inducing a meiosis-like division in a somatic genome, which could one day help women without functional eggs have genetically related children.
"Infertility affects millions of individuals worldwide and is often caused by the absence of functional gametes," the researchers wrote. For women, age-related decline in egg quality becomes a primary factor after the mid-thirties, and current fertility treatments cannot help those who lack viable eggs altogether.
How Skin Cells Became Egg-Like Structures
The process takes a skin cell's nucleus and inserts it into a donated egg cell that has had its own genetic material removed, a technique called somatic cell nuclear transfer or SCNT. Scientists have used this method for decades to clone animals, but the Oregon team adapted it for a different purpose: creating reconstructed cells that could potentially be fertilized like natural eggs.
When a skin cell nucleus enters the donor egg environment, something unexpected occurs. The egg cytoplasm, which provides the cellular machinery needed for division, forces the skin cell's chromosomes to organize themselves into a spindle structure similar to what occurs in natural eggs. Within about two hours after the transfer, visible spindles formed in about 77% of the reconstructed cells.
This happens even though skin cells have not undergone the DNA replication that normally precedes cell division. Natural eggs contain duplicated chromosomes, each consisting of two connected copies called sister chromatids. The skin cell chromosomes, by contrast, are single, non-duplicated copies. Yet the donor egg cytoplasm coaxes them into forming a division-ready structure anyway.



