
Breaking News
 Pentagon Partners With xAI Service For Military's Growing Artificial Intelligence Toolset
Pentagon Partners With xAI Service For Military's Growing Artificial Intelligence Toolset
 Pharmakeia: America's Seniors Are Being Overmedicated Into Oblivion
Pharmakeia: America's Seniors Are Being Overmedicated Into Oblivion
 The New Battle for the Americas: Why the Western Hemisphere Is Becoming a Global...
The New Battle for the Americas: Why the Western Hemisphere Is Becoming a Global...
Top Tech News
 Travel gadget promises to dry and iron your clothes – totally hands-free
Travel gadget promises to dry and iron your clothes – totally hands-free
 Perfect Aircrete, Kitchen Ingredients.
Perfect Aircrete, Kitchen Ingredients.
 Futuristic pixel-raising display lets you feel what's onscreen
Futuristic pixel-raising display lets you feel what's onscreen
 Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
 A microbial cleanup for glyphosate just earned a patent. Here's why that matters
A microbial cleanup for glyphosate just earned a patent. Here's why that matters
 Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Thanks to AI, Computers Can Now See Your Health Problems
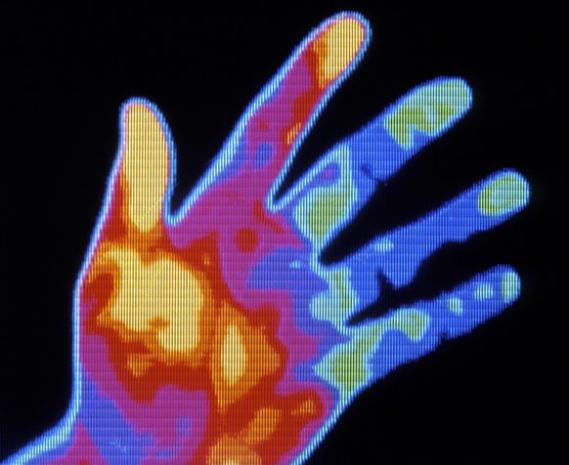
Patient Number Two was born to first-time parents, late 20s, white. The pregnancy was normal and the birth uncomplicated. But after a few months, it became clear something was wrong. The child had ear infection after ear infection and trouble breathing at night. He was small for his age, and by his fifth birthday, still hadn't spoken. He started having seizures. Brain MRIs, molecular analyses, basic genetic testing, scores of doctors; nothing turned up answers. With no further options, in 2015 his family decided to sequence their exomes—the portion of the genome that codes for proteins—to see if he had inherited a genetic disorder from his parents. A single variant showed up: ARID1B.
The mutation suggested he had a disease called Coffin-Siris syndrome. But Patient Number Two didn't have that disease's typical symptoms, like sparse scalp hair and incomplete pinky fingers. So, doctors, including Karen Gripp, who met with Two's family to discuss the exome results, hadn't really considered it. Gripp was doubly surprised when she uploaded a photo of Two's face to Face2Gene. The app, developed by the same programmers who taught Facebook to find your face in your friend's photos, conducted millions of tiny calculations in rapid succession—how much slant in the eye? How narrow is that eyelid fissure? How low are the ears? Quantified, computed, and ranked to suggest the most probable syndromes associated with the facial phenotype. There's even a heat map overlay on the photo that shows which the features are the most indicative match.
"In hindsight it was all clear to me," says Gripp, who is chief of the Division of Medical Genetics at A.I. duPont Hospital for Children in Delaware, and had been seeing the patient for years. "But it hadn't been clear to anyone before." What had taken Patient Number Two's doctors 16 years to find took Face2Gene just a few minutes.

FDNA
Face2Gene takes advantage of the fact that so many genetic conditions have a tell-tale "face"—a unique constellation of features that can provide clues to a potential diagnosis. It is just one of several new technologies taking advantage of how quickly modern computers can analyze, sort, and find patterns across huge reams of data. They are built in fields of artificial intelligence known as deep learning and neural nets—among the most promising to deliver AI's 50-year old promise to revolutionize medicine by recognizing and diagnosing disease.
Genetic syndromes aren't the only diagnoses that could get help from machine learning. The RightEye GeoPref Autism Test can identify the early stages of autism in infants as young as 12 months—the crucial stages where early intervention can make a big difference.

 No Excuses: Throw A Party!
No Excuses: Throw A Party!


