
Breaking News
 Silver up over $2.26... Today! $71.24 (and Gold close to $4500)
Silver up over $2.26... Today! $71.24 (and Gold close to $4500)
 GARLAND FAVORITO: More and more fraud from the 2020 election in Fulton County, Georgia...
GARLAND FAVORITO: More and more fraud from the 2020 election in Fulton County, Georgia...
 Rep. Matt Gaetz tells Tucker Carlson that agents of the Israeli govt tried to blackmail his...
Rep. Matt Gaetz tells Tucker Carlson that agents of the Israeli govt tried to blackmail his...
 Trump: We need Greenland for national security… you have Russian and Chinese ships all over...
Trump: We need Greenland for national security… you have Russian and Chinese ships all over...
Top Tech News
 Travel gadget promises to dry and iron your clothes – totally hands-free
Travel gadget promises to dry and iron your clothes – totally hands-free
 Perfect Aircrete, Kitchen Ingredients.
Perfect Aircrete, Kitchen Ingredients.
 Futuristic pixel-raising display lets you feel what's onscreen
Futuristic pixel-raising display lets you feel what's onscreen
 Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
 A microbial cleanup for glyphosate just earned a patent. Here's why that matters
A microbial cleanup for glyphosate just earned a patent. Here's why that matters
 Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Nanostructured electrode could boost lithium battery storage by 50%
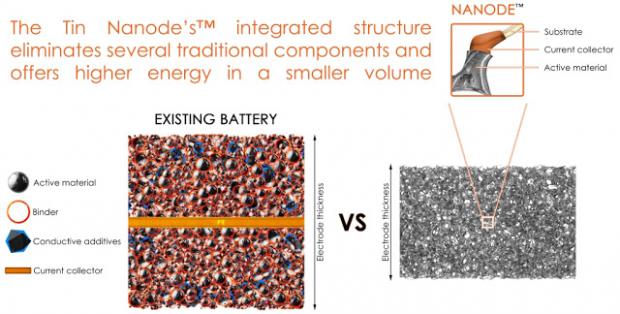
This allows the batteries to last longer between charges while also charging faster. These achievements are due to both the material structure and the use of tin as the active material. Tin is known to have much higher energy density than the current graphite technology, but until now its commercial success has been limited due to its tendency to swell during charging, causing stress in the electrode material and leading to a rapid loss in energy. Current commercial lithium ion batteries employ a foil/particle system as the electrode structure. The capability of such electrodes to deal with volume expansion of high energy materials is limited, because as the particles swell, the electrode expands.
The Tin Nanode's™ integrated electrode structure contributes to the relaxation of stress associated with electrode materials undergoing high volume expansion. This is possible because thin films of active material are spread over a 3D and porous network of fibres, rather than stacking particles on a flat copper foil.



