
Breaking News
 Japanese biologist Yoshinori Ohsumi won the Nobel Prize for discovering how the body...
Japanese biologist Yoshinori Ohsumi won the Nobel Prize for discovering how the body...
 IT BYPASSES THE RETINA. IT BYPASSES THE OPTIC NERVE. IT SENDS IMAGES STRAIGHT...
IT BYPASSES THE RETINA. IT BYPASSES THE OPTIC NERVE. IT SENDS IMAGES STRAIGHT...
 PROMOTING THE NATIONAL DEFENSE BY ENSURING AN ADEQUATE SUPPLY OF...
PROMOTING THE NATIONAL DEFENSE BY ENSURING AN ADEQUATE SUPPLY OF...
 REAL-ID, Mail-Order CDLs, and America's CDL Free-for-All.
REAL-ID, Mail-Order CDLs, and America's CDL Free-for-All.
Top Tech News
 New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
 AI-enhanced stethoscope excels at listening to our hearts
AI-enhanced stethoscope excels at listening to our hearts
 Flame-treated sunscreen keeps the zinc but cuts the smeary white look
Flame-treated sunscreen keeps the zinc but cuts the smeary white look
 Display hub adds three more screens powered through single USB port
Display hub adds three more screens powered through single USB port
 We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
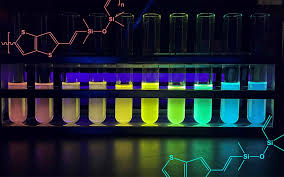
Insulator Becomes Conducting Semiconductor And Could Make Superelastic Silicone Solar Panels
On a molecular level, silicones are made up of a backbone of alternating silicon and oxygen atoms (Si—O—Si) with organic (carbon-based) groups attached to the silicon. Various 3D formations of polymer chains arise as they connect to one another, known as cross-linking, which alter the material's physical properties like strength or solubility.
This could lead to super-elastic silicone PV cells(/panels) and super-elastic thus super-durable, thick and highly energy dense lithium-ion battery anodes.
While studying different cross-linking structures in silicone, the research team stumbled upon the potential for electrical conductivity in a copolymer, which is a polymer chain containing two different types of repeating units—cage-structured and then linear silicones in this case.
The possibility for conductivity arises from the way electrons can move across Si—O—Si bonds with overlapping orbitals. Semiconductors have two main states: the ground state, which doesn't conduct electricity, and a conducting state, which does. The conducting state, also known as an excited state, occurs when some electrons jump up to the next electron orbital, which is connected across the material like a metal.
Typically, Si—O—Si bond angles don't allow for that connection. At 110°, they are a long way from a 180° straight line. But in the silicone copolymer the team discovered, these bonds started out at 140° in the ground state—and they stretch to 150° in the excited state. This was enough to create a highway for electrical charge to flow.
σ–σ* conjugation Across Si?O?Si Bonds. Macromol.
"This allows an unexpected interaction between electrons across multiple bonds including Si—O—Si bonds in these copolymers," Laine said. "The longer the chain length, the easier it is for electrons to travel longer distances, reducing the energy needed to absorb light and then emit it at lower energies."
The semiconducting properties of the silicone copolymers also enable its spectrum of colors. Electrons jump between the ground and excited states by absorbing and emitting photons, or particles of light. The light emission depends on the length of the copolymer chain, which Laine's team can control. Longer chain lengths mean smaller jumps and lower energy photons, giving the silicone a red tint. Shorter chains require bigger jumps from the electrons, so they emit higher energy light toward the blue end of the spectrum.



