
Breaking News
 Verizon to Slash 15 Percent of Its Workforce, About 15,000 Jobs
Verizon to Slash 15 Percent of Its Workforce, About 15,000 Jobs
 The SCARY SHIFT from SCARCITY to ABUNDANCE
The SCARY SHIFT from SCARCITY to ABUNDANCE
 Foreclosure surge signals housing market distress as costs spiral out of control
Foreclosure surge signals housing market distress as costs spiral out of control
 Nearly 1,000 flights canceled despite government reopening, disruptions expected for days
Nearly 1,000 flights canceled despite government reopening, disruptions expected for days
Top Tech News
 Blue Origin New Glenn 2 Next Launch and How Many Launches in 2026 and 2027
Blue Origin New Glenn 2 Next Launch and How Many Launches in 2026 and 2027
 China's thorium reactor aims to fuse power and parity
China's thorium reactor aims to fuse power and parity
 Ancient way to create penicillin, a medicine from ancient era
Ancient way to create penicillin, a medicine from ancient era
 Goodbye, Cavities? Scientists Just Found a Way to Regrow Tooth Enamel
Goodbye, Cavities? Scientists Just Found a Way to Regrow Tooth Enamel
 Scientists Say They've Figured Out How to Transcribe Your Thoughts From an MRI Scan
Scientists Say They've Figured Out How to Transcribe Your Thoughts From an MRI Scan
 SanDisk stuffed 1 TB of storage into the smallest Type-C thumb drive ever
SanDisk stuffed 1 TB of storage into the smallest Type-C thumb drive ever
 Calling Dr. Grok. Can AI Do Better than Your Primary Physician?
Calling Dr. Grok. Can AI Do Better than Your Primary Physician?
 HUGE 32kWh LiFePO4 DIY Battery w/ 628Ah Cells! 90 Minute Build
HUGE 32kWh LiFePO4 DIY Battery w/ 628Ah Cells! 90 Minute Build
 What Has Bitcoin Become 17 Years After Satoshi Nakamoto Published The Whitepaper?
What Has Bitcoin Become 17 Years After Satoshi Nakamoto Published The Whitepaper?
A Brilliant New Cancer Treatment That Re-Engineers Human Cells Just Got Approved
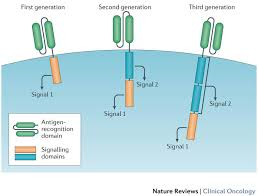
The US Food and Drug Administration (FDA) just approved a cutting-edge cancer therapy.
On Wednesday, the FDA approved Novartis's Kymriah, also known as tisagenlecleucel, a treatment for pediatric acute lymphoblastic lymphoblastic leukemia.
"I think this is most exciting thing I've seen in my lifetime," Dr. Tim Cripe, an oncologist who was part of the FDA advisory committee panel that voted in favour of approving the drug in July.
The highly personalised treatment is called CAR T-cell therapy. It's a type of cancer immunotherapy — or a therapy that harnesses the body's immune system to take on cancer cells.
"We're entering a new frontier in medical innovation with the ability to reprogram a patient's own cells to attack a deadly cancer," FDA commissioner Scott Gottlieb said in a statement.

 Unbanked In A Connected World
Unbanked In A Connected World

