
Breaking News
 Battleborn Batteries Responds! Their Overheating Device is a "Feature" not a "Problem
Battleborn Batteries Responds! Their Overheating Device is a "Feature" not a "Problem
 Actor Liam Neeson Outs Himself as MAHA After Narrating Pro-RFK Jr. Documentary Slamming...
Actor Liam Neeson Outs Himself as MAHA After Narrating Pro-RFK Jr. Documentary Slamming...
 Kyle Rittenhouse announced on social media Wednesday that he has tied the knot.
Kyle Rittenhouse announced on social media Wednesday that he has tied the knot.
 JUST IN: President Trump Grants Tina Peters Pardon
JUST IN: President Trump Grants Tina Peters Pardon
Top Tech News
 Build a Greenhouse HEATER that Lasts 10-15 DAYS!
Build a Greenhouse HEATER that Lasts 10-15 DAYS!
 Look at the genius idea he came up with using this tank that nobody wanted
Look at the genius idea he came up with using this tank that nobody wanted
 Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
 Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
 Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
 We've wiretapped the gut-brain hotline to decode signals driving disease
We've wiretapped the gut-brain hotline to decode signals driving disease
 3D-printable concrete alternative hardens in three days, not four weeks
3D-printable concrete alternative hardens in three days, not four weeks
 Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
Tissue engineering, replacement organs and regenerative medicine are getting friendlier regulations
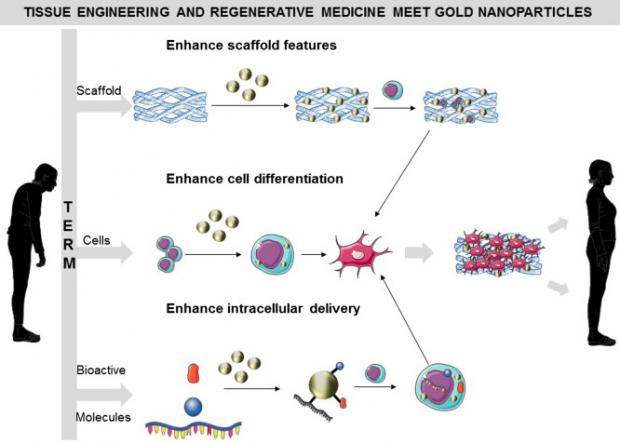
The FDA intends to promote the "least burdensome" rules for companies big and small that are seeking to develop new therapies, "while ensuring patient safety."
"Our policy will allow product manufacturers that time to engage with the FDA to determine if they need to submit a marketing authorization application and, if so, seek guidance on how to submit their application to the FDA for approval," Gottlieb said.
The new rules are in keeping with provisions from the 21st Century Cures Act, passed by Congress in December. That legislation earmarked $6.3 billion in funding, mostly for the U.S. National Institutes of Health, towards groundbreaking medical research.
Over the past few years scientists and physicians have developed tissue-engineered skin for transplant; bladders grown from a patient's own cells; and tissues grown to repair ailing hearts or failing knees.

 First totally synthetic human brain model has been realized
First totally synthetic human brain model has been realized Mach-23 potato gun to shoot satellites into space
Mach-23 potato gun to shoot satellites into space

