
Breaking News
 "You Must Prepare Now" - Doug Casey
"You Must Prepare Now" - Doug Casey
 Will the BRICS 'UNIT' really challenge the dollar?
Will the BRICS 'UNIT' really challenge the dollar?
 The U.S. Just Launched a Secret Dollar Empire (And Nobody Noticed)
The U.S. Just Launched a Secret Dollar Empire (And Nobody Noticed)
 ICE Uses a Growing Web of AI Services to Power Its Immigration Enforcement and Surveillance
ICE Uses a Growing Web of AI Services to Power Its Immigration Enforcement and Surveillance
Top Tech News
 EngineAI T800: Born to Disrupt! #EngineAI #robotics #newtechnology #newproduct
EngineAI T800: Born to Disrupt! #EngineAI #robotics #newtechnology #newproduct
 This Silicon Anode Breakthrough Could Mark A Turning Point For EV Batteries [Update]
This Silicon Anode Breakthrough Could Mark A Turning Point For EV Batteries [Update]
 Travel gadget promises to dry and iron your clothes – totally hands-free
Travel gadget promises to dry and iron your clothes – totally hands-free
 Perfect Aircrete, Kitchen Ingredients.
Perfect Aircrete, Kitchen Ingredients.
 Futuristic pixel-raising display lets you feel what's onscreen
Futuristic pixel-raising display lets you feel what's onscreen
 Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
Cutting-Edge Facility Generates Pure Water and Hydrogen Fuel from Seawater for Mere Pennies
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
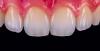 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
IBM Quantum Computer to Help Develop Next-gen Lithium Sulfur Batteries

A quantum computing breakthrough by researchers at IBM and Daimler AG, the parent company of Mercedes-Benz, uses a quantum computer to model the dipole moment of three lithium-containing molecules, which brings us one step closer the next-generation lithium sulfur (Li-S) batteries that would be more powerful, longer lasting and cheaper than today's widely used lithium ion batteries.
Simulating molecules is extremely difficult but modeling them precisely is crucial to discover new drugs and materials. In the research paper "Quantum Chemistry Simulations of Dominant Products in Lithium-Sulfur Batteries," we simulated the ground state energies and the dipole moments of the molecules that could form in lithium-sulfur batteries during operation: lithium hydride (LiH), hydrogen sulfide (H2S), lithium hydrogen sulfide (LiSH), and the desired product, lithium sulfide (Li2S). In addition, and for the first time ever on quantum hardware, we demonstrated that we can calculate the dipole moment for LiH using 4 qubits on IBM Q Valencia, a premium-access 5-qubit quantum computer.
Arxiv- Quantum Chemistry Simulations of Dominant Products in Lithium-Sulfur Batteries



