
Breaking News
Part 4: Immigration Is Killing America: Here Are The Results Coming
 2026-03-05 Ernest Hancock interviews Dr Phranq Tamburri (Trump Report) MP3 (MP4 to be loaded shortly
2026-03-05 Ernest Hancock interviews Dr Phranq Tamburri (Trump Report) MP3 (MP4 to be loaded shortly
 S3E8: Your Money, Your Data, Your Blood, All Stolen
S3E8: Your Money, Your Data, Your Blood, All Stolen
 The Pentagon is looking for the SpaceX of the ocean.
The Pentagon is looking for the SpaceX of the ocean.
Top Tech News
 The Pentagon is looking for the SpaceX of the ocean.
The Pentagon is looking for the SpaceX of the ocean.
 Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
 Scientists at Rice University have developed an exciting new two-dimensional carbon material...
Scientists at Rice University have developed an exciting new two-dimensional carbon material...
 Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
 ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
 DARPA Launches New Program Generative Optogenetics, GO,...
DARPA Launches New Program Generative Optogenetics, GO,...
 Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
 Ordering a Tiny House from China, what's the real COST?
Ordering a Tiny House from China, what's the real COST?
 New video may offer glimpse of secret F-47 fighter
New video may offer glimpse of secret F-47 fighter
 Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Progress to Practical Room Temperature Superconductors
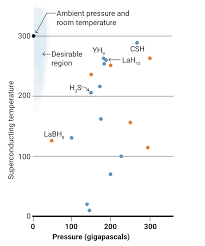
Theorists have predicted other hydride compounds which could work at lower pressures. There is race to find versions stable at ambient pressure and room temperature.
In 2004, Ashcroft suggested that adding other elements to hydrogen might add a "chemical precompression," stabilizing the hydrogen lattice at lower pressures. The race was on to make superconducting hydrides. In 2015, researchers including Mikhail Eremets, a physicist at the Max Planck Institute for Chemistry, reported in Nature that a mix of sulfur and hydrogen superconducted at 203 K when pressurized to 155 GPa. Over the next 3 years, Eremets and others boosted the Tc as high as 250 K in hydrides containing the heavy metal lanthanum. Then came Dias's CSH compound, reported late last year in Nature, which superconducts at 287 K—or 14°C, the temperature of a wine cellar—under 267 GPa of pressure, followed by an yttrium hydride that superconducts at nearly as warm a temperature, announced by multiple groups this year.



