
Breaking News
 Smotrich: 'In the End,' Israel Will Occupy Gaza and Establish Jewish Settlements
Smotrich: 'In the End,' Israel Will Occupy Gaza and Establish Jewish Settlements
 70% of the food that we eat is ultra-processed. It's not really food - it's poison.
70% of the food that we eat is ultra-processed. It's not really food - it's poison.
 No one had more insider information than Jeffrey Epstein,...
No one had more insider information than Jeffrey Epstein,...
 What's REALLY Behind Mexico's Cartel Wars?
What's REALLY Behind Mexico's Cartel Wars?
Top Tech News
 New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
 AI-enhanced stethoscope excels at listening to our hearts
AI-enhanced stethoscope excels at listening to our hearts
 Flame-treated sunscreen keeps the zinc but cuts the smeary white look
Flame-treated sunscreen keeps the zinc but cuts the smeary white look
 Display hub adds three more screens powered through single USB port
Display hub adds three more screens powered through single USB port
 We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
Bio-compatible Glue For Repairing Corneas – A Sight for Sore Eyes
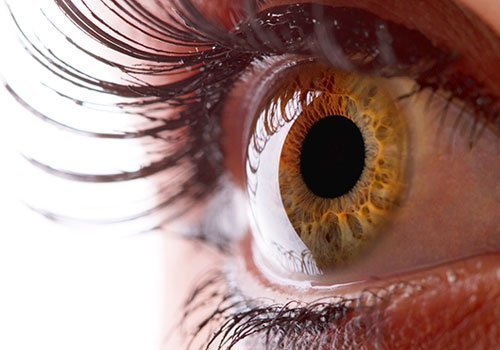
An assistant professor of bioengineering at the University of California-Riverside has developed an adhesive that rapidly integrates corneal cells to speed healing and reduce the chances of secondary infection.
The breakthrough by Iman Noshadi also reduces complications that increase the risk, cost, and healing time of eye injuries and corneal transplants.
Of the 2.4 million eye injuries annually in the United States, 600,000 are open globe corneal tears, leading to blindness in some cases. There are also around 40,000 corneal grafting procedures performed each year.
The current standards of care for corneal repair are sutures and cyanoacrylate glue, both of which are associated with complications and costs, during and after the procedure.
Sutures, used for larger tears and grafting, can cause astigmatism, vascularization, and infections. Cyanoacrylate glue, which is not FDA approved, can be used only for tiny perforations, has low biocompatibility, cytotoxicity, opacity, and causes infections and cataract – all needing additional care and cost.
"There is no FDA-approved material platform to replace the present techniques for corneal repair effectively," said Noshadi. "Our technology addresses all these issues, resulting in reduced operative and post-operative costs."
The UC Riverside product should solve these secondary problems with its biocompatibility, high adhesion, flexibility, and high strength. It will not accidentally loosen or fall off and its antimicrobial properties will help prevent infections. Because the adhesive is transparent, it will help restore vision more quickly, as well.



