
Breaking News
 Free Ian Freeman: Defend Financial Privacy and Justice - Sign the petition
Free Ian Freeman: Defend Financial Privacy and Justice - Sign the petition
 Charlie Robinson with Tim James, Marjory Wildcraft & Patrick Henningsen:
Charlie Robinson with Tim James, Marjory Wildcraft & Patrick Henningsen:
 It's Time To Reopen The Franklin Child Prostitution Case After Epstein Revelations
It's Time To Reopen The Franklin Child Prostitution Case After Epstein Revelations
Top Tech News
 New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
New Spray-on Powder Instantly Seals Life-Threatening Wounds in Battle or During Disasters
 AI-enhanced stethoscope excels at listening to our hearts
AI-enhanced stethoscope excels at listening to our hearts
 Flame-treated sunscreen keeps the zinc but cuts the smeary white look
Flame-treated sunscreen keeps the zinc but cuts the smeary white look
 Display hub adds three more screens powered through single USB port
Display hub adds three more screens powered through single USB port
 We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
We Finally Know How Fast The Tesla Semi Will Charge: Very, Very Fast
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
U.K. approves CRISPR gene editing to create GMO humans
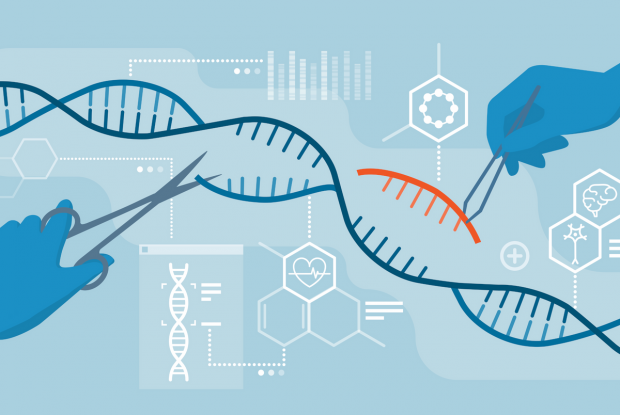
The gene-editing therapy, which was reportedly designed to treat blood disorders, could appear on the market by as soon as December.
The way CRISPR works is that it functions like a pair of "genetic scissors," chopping up sections of DNA so scientists can edit them by "snipping" specific portions and replacing those portions with new, artificial segments.
According to a 2012 paper that first announced the technology, CRISPR is basically a cheap and easy way to edit genes and create new genetically modified organisms (GMOs) – in this case, out of human beings.
Known as exa-cel therapy, the new CRISPR about to be released, known formally as Casgevy, is the world's first CRISPR therapy for humans to receive approval on the market.
CRISPR's inventers won a Nobel Prize in chemistry in 2020 for the discovery, which in recent years has been used to manipulate plants as a possible solution to "climate change." Only now is CRISPR about to be used on humans, despite the fact that it is highly risky.
Is genetically modifying your body really worth a "cure?"
Casgevy was designed to treat two specific blood conditions: sickle cell disease and beta thalassemia. Also known as sickle cell anemia, the former most commonly occurs in people of African or Caribbean descent and is known to cause debilitating pain.
Beta thalassemia, which can cause mild or severe anemia, is a similar condition that typically requires regular blood transfusions to treat.
Both of these genetic conditions are said to be caused by "errors" in the genes that regulate hemoglobin, a protein that lets red blood cells transport oxygen around the body. Both conditions can also be fatal if left untreated.
Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, the technology was approved after just one study that followed only 29 out of 45 total participants for 16 months. Twenty-eight out of these 29 supposedly had no pain after one year, Nature reported.

 The Question Not Being Asked
The Question Not Being Asked

