
Breaking News
 Enoch AI: The first unbiased machine cognition model defying big pharma narratives
Enoch AI: The first unbiased machine cognition model defying big pharma narratives
 BREAKING EXCLUSIVE: President Trump Leverages Massive New Tariffs Against BRICS Countries...
BREAKING EXCLUSIVE: President Trump Leverages Massive New Tariffs Against BRICS Countries...
 California Might Stop Making Necessary Debt Payments For 2 Years
California Might Stop Making Necessary Debt Payments For 2 Years
 US Orders "Immediate Shutdown" Of Mexican Cattle Trade After Cross-Border Parasitic Fly Th
US Orders "Immediate Shutdown" Of Mexican Cattle Trade After Cross-Border Parasitic Fly Th
Top Tech News
Magic mushrooms may hold the secret to longevity: Psilocybin extends lifespan by 57%...
 Unitree G1 vs Boston Dynamics Atlas vs Optimus Gen 2 Robot– Who Wins?
Unitree G1 vs Boston Dynamics Atlas vs Optimus Gen 2 Robot– Who Wins?
 LFP Battery Fire Safety: What You NEED to Know
LFP Battery Fire Safety: What You NEED to Know
 Final Summer Solar Panel Test: Bifacial Optimization. Save Money w/ These Results!
Final Summer Solar Panel Test: Bifacial Optimization. Save Money w/ These Results!
 MEDICAL MIRACLE IN JAPAN: Paralyzed Man Stands Again After Revolutionary Stem Cell Treatment!
MEDICAL MIRACLE IN JAPAN: Paralyzed Man Stands Again After Revolutionary Stem Cell Treatment!
 Insulator Becomes Conducting Semiconductor And Could Make Superelastic Silicone Solar Panels
Insulator Becomes Conducting Semiconductor And Could Make Superelastic Silicone Solar Panels
 Slate Truck's Under $20,000 Price Tag Just Became A Political Casualty
Slate Truck's Under $20,000 Price Tag Just Became A Political Casualty
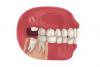 Wisdom Teeth Contain Unique Stem Cell That Can Form Cartilage, Neurons, and Heart Tissue
Wisdom Teeth Contain Unique Stem Cell That Can Form Cartilage, Neurons, and Heart Tissue
 Hay fever breakthrough: 'Molecular shield' blocks allergy trigger at the site
Hay fever breakthrough: 'Molecular shield' blocks allergy trigger at the site
Moderna's COVID-19 Vaccine Spikevax Receives Full FDA Approval for Children...
The U.S. Food and Drug Administration (FDA) has officially granted full approval to Moderna's controversial mRNA COVID-19 vaccine, Spikevax, for children as young as 6 months old through 11 years of age who are deemed "at increased risk" of COVID-19.
The vaccine was previously available to kids under Emergency Use Authorization (EUA), but the FDA has now handed over full approval, greenlighting the use of the experimental shot despite growing evidence of adverse events, including myocarditis, pericarditis, allergic reactions, and even seizures in young recipients.
"COVID-19 continues to pose a significant potential threat to children, especially those with underlying medical conditions. Vaccination can be an important tool for protecting our youngest against severe disease and hospitalization," said Stéphane Bancel, Chief Executive Officer of Moderna.
"We appreciate the FDA's diligent scientific review and approval of Spikevax for pediatric populations at increased risk for COVID-19 disease."
While the media parrots talking points about "protecting vulnerable children," the fine print tells a different story:
Myocarditis and pericarditis risks are highest in males ages 12 to 24—and Moderna wants to jab children as young as 6 months.
Adverse reactions include: seizures, chest pain, shortness of breath, vomiting, and even fainting.
Parents are told to "stay nearby for up to an hour" after their child's injection—just in case their toddler has a severe allergic reaction or passes out.
The vaccine may not protect all recipients, yet Moderna continues pushing forward with mass vaccination.

 AI Getting Better at Medical Diagnosis
AI Getting Better at Medical Diagnosis

