
Breaking News
 BREAKING: CBS 60 Minutes: revealed a previously unknown weapon that they believe is linked...
BREAKING: CBS 60 Minutes: revealed a previously unknown weapon that they believe is linked...
 The Year of Adam Smith: Why the Savvy Scotsman Remains So Important
The Year of Adam Smith: Why the Savvy Scotsman Remains So Important
 Trump sons trigger 'corruption' uproar as Pentagon drone venture surfaces amid Iran war
Trump sons trigger 'corruption' uproar as Pentagon drone venture surfaces amid Iran war
 Will the Dollar be a Casualty of the Iran War?
Will the Dollar be a Casualty of the Iran War?
Top Tech News
 The Pentagon is looking for the SpaceX of the ocean.
The Pentagon is looking for the SpaceX of the ocean.
 Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
 Scientists at Rice University have developed an exciting new two-dimensional carbon material...
Scientists at Rice University have developed an exciting new two-dimensional carbon material...
 Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
 ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
 DARPA Launches New Program Generative Optogenetics, GO,...
DARPA Launches New Program Generative Optogenetics, GO,...
 Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
 Ordering a Tiny House from China, what's the real COST?
Ordering a Tiny House from China, what's the real COST?
 New video may offer glimpse of secret F-47 fighter
New video may offer glimpse of secret F-47 fighter
 Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Adding silicon-sulfur into 3D graphene makes for game-changing battery potential
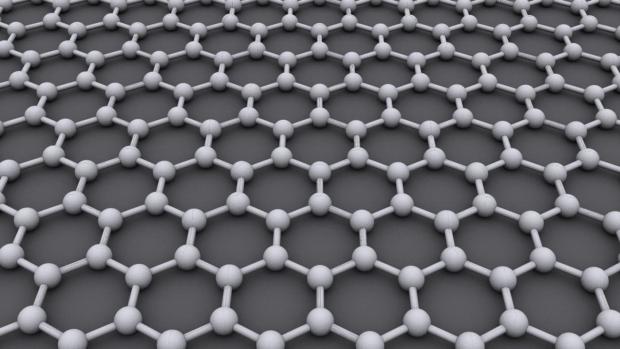
Chemists have long known that lithium-sulfur has huge potential as a next-generation battery solution, combining the strengths of a fuel cell (very energy dense) with the strengths of a battery (self-contained energy storage) – all in a package that is extremely environmentally-friendly and that has a low cost of manufacture.
The problem is that cathodes of sulfur and lithium have lots of material loss due to the solubility of polysulfides, and are not often efficient because sulfur has insulative properties rather than conductive. Arranging the sulfur in the lithium mix via various methods has previously shown promise, but has strict limits that have so far not allowed Li-S batteries to be viable for commercialization.
Various attempts to control the sulfur within the lithium mix have usually centered on porous carbons (usually activated carbon) for macroporous, mesoporous, and microporous solutions to make carbon-sulfur hybrids. These have worked, to a point, but have restricted pore volumes and thus limited viability. Likewise, sulfur copolymers have been a promising choice, but still have conductivity issues.



