
Breaking News
 HERE'S WHAT NO CASH ACTUALLY MEANS (Dave Ramsey re-post)
HERE'S WHAT NO CASH ACTUALLY MEANS (Dave Ramsey re-post)
 The Silver Shift: Why Stackers Are DUMPING 90% Silver & Buying SilverBitz!
The Silver Shift: Why Stackers Are DUMPING 90% Silver & Buying SilverBitz!
 Eye-bouncing - #SolutionsWatch
Eye-bouncing - #SolutionsWatch
 'Targeted, Antisemitism': 16 Dead, 38 Injured After Father & Son Terrorists Attack...
'Targeted, Antisemitism': 16 Dead, 38 Injured After Father & Son Terrorists Attack...
Top Tech News
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
 A microbial cleanup for glyphosate just earned a patent. Here's why that matters
A microbial cleanup for glyphosate just earned a patent. Here's why that matters
 Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
 Advanced Propulsion Resources Part 1 of 2
Advanced Propulsion Resources Part 1 of 2
 PulsarFusion a forward-thinking UK aerospace company, is pushing the boundaries of space travel...
PulsarFusion a forward-thinking UK aerospace company, is pushing the boundaries of space travel...
 Dinky little laser box throws big-screen entertainment from inches away
Dinky little laser box throws big-screen entertainment from inches away
 'World's first' sodium-ion flashlight shines bright even at -40 ºF
'World's first' sodium-ion flashlight shines bright even at -40 ºF
Scientists accidentally create nanorods that harvest water from the air
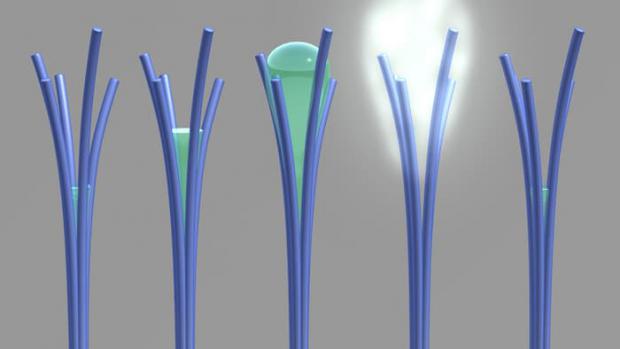
After unintentionally creating carbon-rich nanorods, the team realized its accidental invention behaves weirdly with water, demonstrating a 20-year old theory and potentially paving the way to low-energy water harvesting systems and sweat-removing fabrics.
The researchers note that ordinarily materials will absorb more water as the humidity in the air around them increases. But between 50 and 80 percent relative humidity, these nanorods will actually do the opposite and expel water, a behavior they say is not shared by any other material. Below that range, they behave as normal, so the process is reversible by lowering the humidity again.
"Our unusual material behaves a bit like a sponge; it wrings itself out halfway before it's fully saturated with water," says David Lao, PNNL research associate and creator of the material.



