
Breaking News
 Argentina Moves to Let Banks Offer Bitcoin and Crypto Services
Argentina Moves to Let Banks Offer Bitcoin and Crypto Services
 We're One Storm Away From Disaster
We're One Storm Away From Disaster
 Think a Dairy Cow Will Tie You Down? Here's the Truth.
Think a Dairy Cow Will Tie You Down? Here's the Truth.
 Students challenge auto industry with modular EV you can fix yourself
Students challenge auto industry with modular EV you can fix yourself
Top Tech News
 This tiny dev board is packed with features for ambitious makers
This tiny dev board is packed with features for ambitious makers
 Scientists Discover Gel to Regrow Tooth Enamel
Scientists Discover Gel to Regrow Tooth Enamel
 Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
Vitamin C and Dandelion Root Killing Cancer Cells -- as Former CDC Director Calls for COVID-19...
 Galactic Brain: US firm plans space-based data centers, power grid to challenge China
Galactic Brain: US firm plans space-based data centers, power grid to challenge China
 A microbial cleanup for glyphosate just earned a patent. Here's why that matters
A microbial cleanup for glyphosate just earned a patent. Here's why that matters
 Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
Japan Breaks Internet Speed Record with 5 Million Times Faster Data Transfer
 Advanced Propulsion Resources Part 1 of 2
Advanced Propulsion Resources Part 1 of 2
 PulsarFusion a forward-thinking UK aerospace company, is pushing the boundaries of space travel...
PulsarFusion a forward-thinking UK aerospace company, is pushing the boundaries of space travel...
 Dinky little laser box throws big-screen entertainment from inches away
Dinky little laser box throws big-screen entertainment from inches away
 'World's first' sodium-ion flashlight shines bright even at -40 ºF
'World's first' sodium-ion flashlight shines bright even at -40 ºF
Power dense zinc-manganese power unit as cheap as a car battery
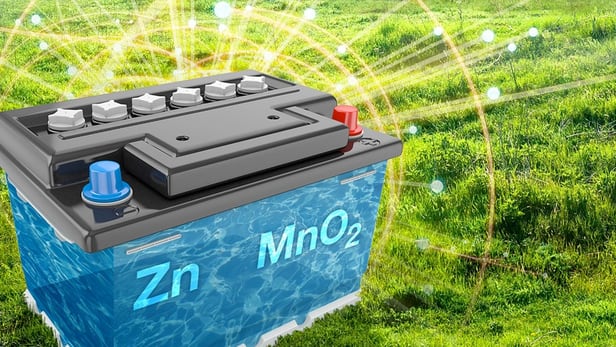
A team of scientists working on analyzing energy flows in prototype zinc-manganese batteries have stumbled upon a new way to make these power cells much more reliable, with many more recharge cycles than the humble lead-acid car battery, but costing around the same to produce. The creators claim that the new battery could become an inexpensive, ecologically-sound alternative for storing energy from renewable sources and a high-density solution for storing excess energy from the power grid.
Working at the Department of Energy's Pacific Northwest National Laboratory (PNNL), the researchers discovered a new way to approach the reliability problems of zinc-manganese batteries, that were cheap and easy to make from abundant materials, but which would fail after only a few charge cycles.
"The idea of a rechargeable zinc-manganese battery isn't new; researchers have been studying them as an inexpensive, safe alternative to lithium-ion batteries since the late 1990s," said PNNL Laboratory Fellow Jun Liu. "But these batteries usually stop working after just a few charges. Our research suggests these failures could have occurred because we failed to control chemical equilibrium in rechargeable zinc-manganese energy storage systems."



