
Breaking News
 Pentagon To Send 200 Troops to Nigeria
Pentagon To Send 200 Troops to Nigeria
 Trump Says He May Send Second Aircraft Carrier to Middle East To Prepare for Potential Attack...
Trump Says He May Send Second Aircraft Carrier to Middle East To Prepare for Potential Attack...
 A Market Crash and Recession Are Bullish, Not Bearish
A Market Crash and Recession Are Bullish, Not Bearish
 What Are They Still Hiding? New Epstein Questions Point to a Much Bigger Cover-Up
What Are They Still Hiding? New Epstein Questions Point to a Much Bigger Cover-Up
Top Tech News
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
Breakthrough for rechargeable non-aqueous magnesium-metal battery...
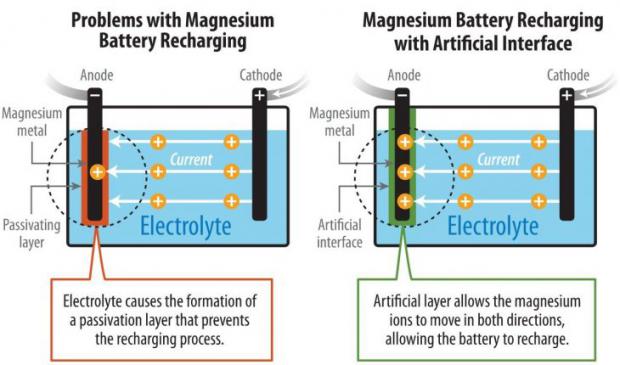
Scientists have pioneered a method to enable the reversible chemistry of magnesium metal in the noncorrosive carbonate-based electrolytes and tested the concept in a prototype cell. The technology possesses potential advantages over lithium-ion batteries—notably, higher energy density, greater stability, and lower cost.
Magnesium (Mg) batteries theoretically contain almost twice as much energy per volume as lithium-ion batteries. But previous research encountered an obstacle: chemical reactions of the conventional carbonate electrolyte created a barrier on the surface of magnesium that prevented the battery from recharging. The magnesium ions could flow in a reverse direction through a highly corrosive liquid electrolyte, but that barred the possibility of a successful high-voltage magnesium battery.



