
Breaking News
 Marjorie Taylor Greene EVISCERATES Trump Over Iran War! w/ Rick Overton
Marjorie Taylor Greene EVISCERATES Trump Over Iran War! w/ Rick Overton
 Sip your way to better gut health with these science-backed, fermented beverages
Sip your way to better gut health with these science-backed, fermented beverages
 The War on Sunlight Is Real (And It's Not an Accident) | Dr. Jack Kruse
The War on Sunlight Is Real (And It's Not an Accident) | Dr. Jack Kruse
 Trump's Unconditional Surrender, NEW Supreme Leader Mojtaba Khamenei + NYC IED Attack...
Trump's Unconditional Surrender, NEW Supreme Leader Mojtaba Khamenei + NYC IED Attack...
Top Tech News
 The Pentagon is looking for the SpaceX of the ocean.
The Pentagon is looking for the SpaceX of the ocean.
 Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
Major milestone by 3D printing an artificial cornea using a specialized "bioink"...
 Scientists at Rice University have developed an exciting new two-dimensional carbon material...
Scientists at Rice University have developed an exciting new two-dimensional carbon material...
 Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
Footage recorded by hashtag#Meta's AI smart glasses is sent to offshore contractors...
 ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
ELON MUSK: "With something like Neuralink… we effectively become maybe one with the AI."
 DARPA Launches New Program Generative Optogenetics, GO,...
DARPA Launches New Program Generative Optogenetics, GO,...
 Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
Anthropic Outpaces OpenAI Revenue 10X, Pentagon vs. Dario, Agents Rent Humans | #234
 Ordering a Tiny House from China, what's the real COST?
Ordering a Tiny House from China, what's the real COST?
 New video may offer glimpse of secret F-47 fighter
New video may offer glimpse of secret F-47 fighter
 Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Donut Lab's Solid-State Battery Charges Fast. But Experts Still Have Questions
Progress to lithium-oxygen batteries with up to four times the energy density of lithium-ion
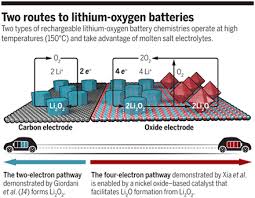
The high theoretical-energy density of lithium-oxygen (Li-O2) batteries and their relatively light weight have made them the Holy Grail of rechargeable battery systems. But long-standing issues with the battery's chemistry and stability have kept them a purely academic curiosity.
Two of the more serious issues involve the intermediate of the cell chemistry (superoxide, LiO2) and the peroxide product (Li2O2) reacting with the porous carbon cathode, degrading the cell from within. In addition, the superoxide consumes the organic electrolyte in the process, which greatly limits the cycle life.



